REDUCE RECOLLECTIONS
Why not eliminate them?
THE SCOPE FOR COLLECTION ERRORS
Managing the sample collection process to ensure that, for each order, all necessary samples are collected, and that each sample is delivered to the lab in viable condition, is a key metric that impacts on diagnostic service quality. The requirement to recollect samples is a general symptom of errors that occur during the pre-analytical process, but the reasons for these errors can be numerous and varied. Recollection of samples, if possible, can be costly to the lab, inconvenient to the patient and can impact the quality of patient care. Samples that cannot be recollected, can pose a serious risk to patient care and treatment.
Currently, most problems occurring during sample collection processes are identified once the sample arrives at the laboratory. Samples may be rejected at the laboratory’s specimen reception, due to incorrect labelling, contamination, collection into an inappropriate anti-coagulant, or because the sample quality is compromised, for example, haemolysed or clotted samples. Problems may also be detected by referring doctors on receipt of results. A doctor may realise that the test order has been incorrectly interpreted, the patient incorrectly identified, incorrect or insufficient tests have been performed, or the results reported are abnormal for the patient. In either case, determining that sample recollection is required, usually occurs late in the testing process, resulting in added cost to the laboratory and inconvenience to patient and ordering doctor.
The opportunities for errors to occur during the pre-analytical process, that can impact the quality of samples collected, remains broad and varied. Samples with identification or quality problems must be rejected by the laboratory when detected. Rejecting problem samples protects the immediate patient safety but ultimately results in sub-optimal patient care and safety, and rework for the laboratory. While laboratories usually record sample errors that they detect as part of their quality management and control system, this does not give a complete picture. Lost or “misplaced” samples are usually not included in the errors counted if the samples are found in time to enable the test to be performed. However, there is a cost to the laboratory in searching for the misplaced samples, to test turn-around time and possible risk to patient safety.
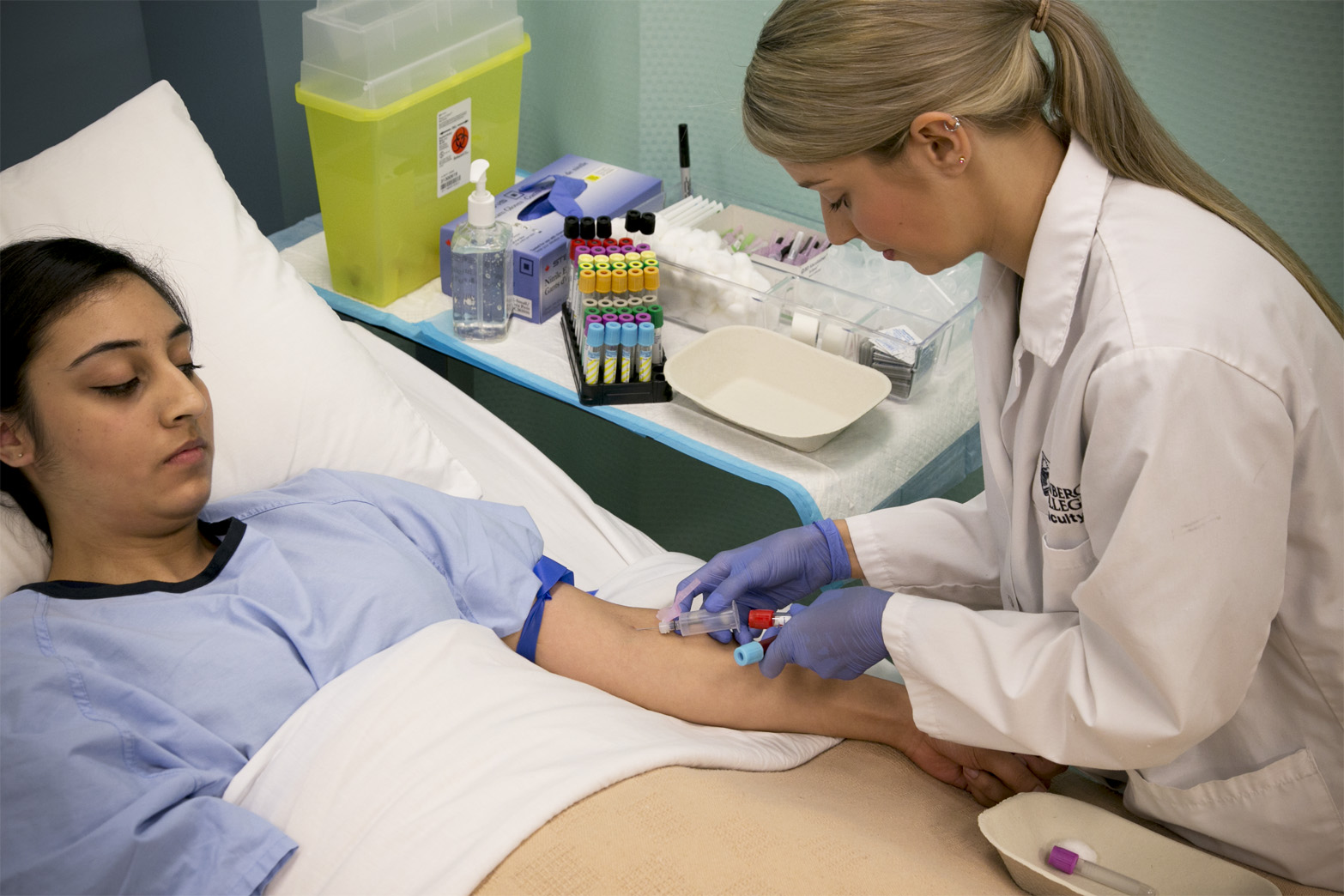
MANAGING COLLECTION QUALITY
Even if recorded, analysing the data to understand the root cause can be the first challenge faced by laboratories. Implementing practices that eliminate sample collection errors, where the cause of sample errors is outside the control of the laboratory can be difficult if not impossible to affect.
Sample collection can be performed in a range of health service settings including public and private hospitals, community-based collection centres, aged care facilities, through home visits and within GP’s rooms. Collections may be undertaken by phlebotomists, nurses, doctors and other healthcare providers. The skills, experience and familiarity of each of these collection personnel, and their understanding of required patient preparation, collection procedures, sample identification, transport and preparation conditions, may vary considerably.
Even within laboratory groups, maintaining a well-trained and qualified phlebotomy workforce remains a challenge, with the often-transient and temporary nature of collection staff. In Australia, which has some of the strongest practices in regard to compliance to quality assurance systems, a significant proportion of the laboratory phlebotomy workforce (estimated to be almost half) remains unqualified. Furthermore, as a significant proportion of collections are undertaken by non-specialist collectors, such as General Practitioners, practice nurses, medical scientists, interns and nurses in specific hospital wards and emergency departments, the potential for errors during the sample collection process is high.
Laboratories have made significant efforts to address this problem – investing in extensive training of their collection staff, the implementation of well-defined collection procedures and strategies for error detection and prevention. While electronic ordering systems can eliminate test interpretation errors, and some patient identification errors, they do not provide the holistic approach that is required to address the scope of sample collection errors that may occur. To eliminate recollections, a dramatic new approach is required that has the scope, and with the reach, to make a significant impact on sample collection and quality control errors.
ASSESSING THE HIDDEN IMPACT

When considering the overall cost of recollection to the laboratory - the cost of maintaining quality control, including staff required to check for and detect sample errors; management of collection procedure manuals, SOP’s and processes; training and competency certification programs, combined with the management overhead of arranging and scheduling recollections, and the potential impact on reputation – the cost to the laboratory can be significant.
Ensuring that each sample collection - regardless of who performs it or where it is performed - is completed correctly is a key step in ensuring that the right, high quality and viable samples are consistently delivered to the laboratory. Understanding the samples required to be collected for each order, and in what order they should be collected, is the first step to ensuring that samples will not have to be recollected. However, this is only the starting point.
Currently, success ultimately depends on vigilance and training of the collector in the field, and their attention to the execution of quality collection procedures. Traditionally, most collections occur without the support of technology, and even with the best care and intentions, human errors will inevitably occur.
A NEW GAME PLAN
PAMS is designed to support each aspect of collections as they are performed. Its key design goal is the elimination of errors from collections. It achieves this by taking advantage of mobile technology to manage key patient preparation, sample collection and handling, and positive patient and sample identification tasks, are completed at point of care, more rigorously and effectively. Rules ensure that the correct samples are collected in the correct order, for every collection activity.
As a mobile application, PAMS can support and manage every step, wherever required and at the point of care. Regardless of skills and expertise, PAMS ensures that appropriate guidance and support is provided to each care provider for every sample collected. PAMS take a holistic approach to the management of every collection activity. From test ordering, scheduling of collections, sample collection, preparation and transport, PAMS manages every aspect of the collection. Every step in the sample collection chain is managed to ensure that the right samples are collected for the right patient, for the right tests to be performed, and that each sample delivered to the lab is viable for the testing to be performed.
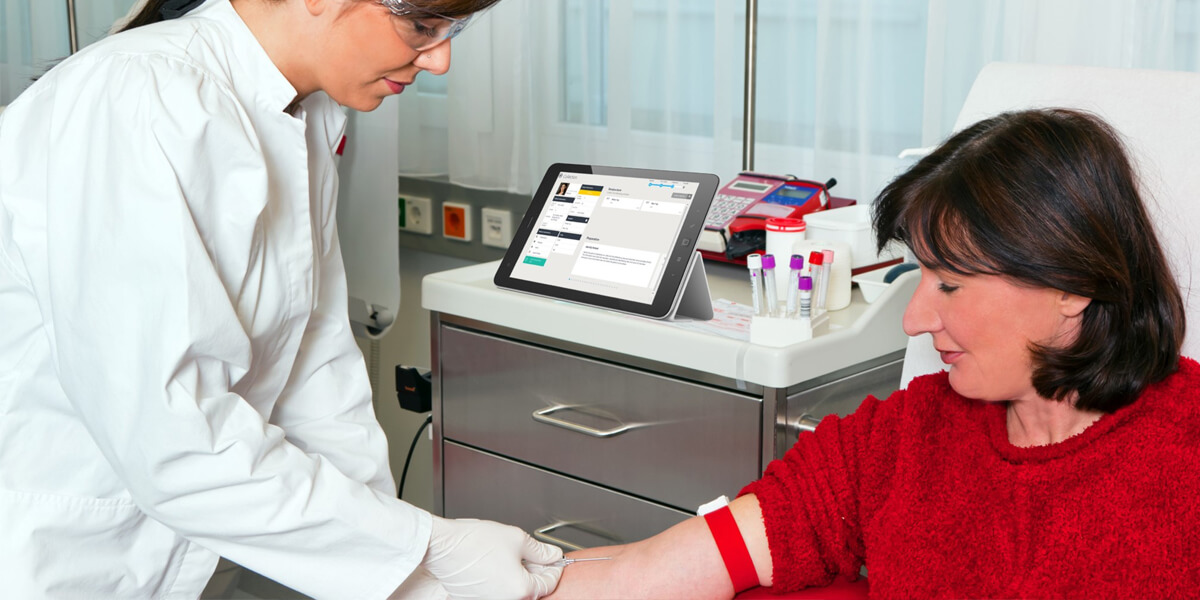
PAMS maintains the connection between ordering clinician, patient and samples to put you in control of every activity required to collect and deliver each sample to the lab. Through real-time monitoring, PAMS enables you to provide the earliest intervention to correct sample anomalies, with visibility that provides a detailed view as to the of cause of anomalies. PAMS collects detailed information for every step in the sample collection process, providing you with the means to fully analyse and understand the causes of collection errors. Uniquely, PAMS then allows you to quickly implement refined procedures and process, training and competency programs to targeted audiences, as integrated parts of the PAMS app suite.
Devices such as tablets and smart phones can be used by doctors and collectors to proactively monitor the entire pre-analytical process. Facilitation of key collection steps such as positive patient and sample identification, guidance on phlebotomy techniques, sample preparation and handling are delivered at the point of care. Authorisation of orders and sample chain of custody is managed through authenticated and secure access control.
PAMS provides the opportunity to completely rethink the methods that can be used to support clinicians, collectors and the laboratory to vastly improve performance of collection activities, so that recollections can be minimised. For the first time, the entire pre-analytical process can be fully integrated. Clinician, collector and laboratory tasks can be supported and monitored so that patient safety concerns can be eliminated. PAMS provides the platform for you to actively manage and control every sample collection activity. PAMS delivers a continuous improvement model with the objective of not just minimising recollections, but to entirely remove recollections caused by preventable errors.